Preclinical evaluation of PHH-1V vaccine candidate against SARS-CoV-2 in non-human primates
Prenafeta, A., Bech-Sàbat, G., Moros, A., Barreiro, A., Fernández, A., Cañete, M., Roca, M., González-González, L., Garriga, C., Confais, J., Toussenot, M., Contamin, H., Pizzorno, A., Rosa-Calatrava, M., Pradenas, E., Marfil, S., Blanco, J., Rica, P. C., Sisteré-Oró, M., Meyerhans, A., Ferrer, L.
iScience. 2023;107224. doi:10.1016/j.isci.2023.107224 [published online ahead of print, 2023 Jun 28].
Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches
Szpiro L, Bourgeay C, Hoareau AL, Julien T, Menard C, Marie Y, Rosa-Calatrava M, Moules V.
Materials. 2023; 16(7):2889. https://doi.org/10.3390/ma16072889
Prior SARS-CoV-2 infection enhances and reshapes spike protein-specific memory induced by vaccination
Barateau V, Peyrot L, Saade C, Pozzetto B, Brengel-Pesce K, Elsensohn MH, Allatif O, Guibert N, Compagnon C, Mariano N, Chaix J, Djebali S, Fassier JB, Lina B, Lefsihane K, Espi M, Thaunat O, Marvel J, Rosa-Calatrava M, Pizzorno A, Maucort-Boulch D, Henaff L, Saadatian-Elahi M, Vanhems P, Paul S, Walzer T, Trouillet-Assant S, Defrance T.
Sci Transl Med. 2023 Mar 15;15(687):eade0550. doi:10.1126/scitranslmed.ade0550. Epub 2023 Mar 15. PMID: 36921035.
Autoantibodies against type I IFNs in patients with critical influenza pneumonia
Zhang Q, Pizzorno A, Miorin L, Bastard P, Gervais A, Le Voyer T, Bizien L, Manry J, Rosain J, Philippot Q, Goavec K, Padey B, Cupic A, Laurent E, Saker K, Vanker M, Särekannu K; COVID Human Genetic Effort; Etablissement Français du Sang Study Group; Constances Cohort; 3C-Dijon Study; Cerba HealthCare Group; Lyon Antigrippe Working Group; REIPI INF Working Group; García-Salum T, Ferres M, Le Corre N, Sánchez-Céspedes J, Balsera-Manzanero M, Carratala J, Retamar-Gentil P, Abelenda-Alonso G, Valiente A, Tiberghien P, Zins M, Debette S, Meyts I, Haerynck F, Castagnoli R, Notarangelo LD, Gonzalez-Granado LI, Dominguez-Pinilla N, Andreakos E, Triantafyllia V, Rodríguez-Gallego C, Solé-Violán J, Ruiz-Hernandez JJ, Rodríguez de Castro F, Ferreres J, Briones M, Wauters J, Vanderbeke L, Feys S, Kuo CY, Lei WT, Ku CL, Tal G, Etzioni A, Hanna S, Fournet T, Casalegno JS, Queromes G, Argaud L, Javouhey E, Rosa-Calatrava M, Cordero E, Aydillo T, Medina RA, Kisand K, Puel A, Jouanguy E, Abel L, Cobat A, Trouillet-Assant S, García-Sastre A, Casanova JL.
J Exp Med. 2022 Nov 7;219(11):e20220514. doi: 10.1084/jem.20220514. Epub 2022 Sep 16. PMID: 36112363.
Interactions between Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Major Respiratory Viruses in Human nasal Epithelium
Pizzorno A, Padey B, Duliere V, Mouton W, Oliva J, Laurent E, Milesi C, Lina B, Traversier A, Julien T, Trouillet-Assant S, Rosa-Caltrava M, Terrier O.
J Infect Dis. 2022 Aug 29:jiac357. doi: 10.1093/infdis/jiac357. Epub ahead of print. PMID: 36031537; PMCID: PMC9452145.
Biotechnological production of sialylated solid lipid microparticles as inhibitors of influenza A virus infection
Richard E, Traversier A, Julien T, Rosa-Calatrava M, Putaux JL, Jeacomine I, Samain E.
Glycobiology. 2022 Aug 24:cwac054. doi: 10.1093/glycob/cwac054. Epub ahead of print. PMID: 36001347.
Treatments for COVID-19: Lessons from 2020 and new therapeutic options.
Salasc F, Lahlali T, Laurent E, Rosa-Calatrava M, Pizzorno A.
[published online ahead of print, 2021 Nov 18]. Curr Opin Pharmacol. 2021;62:43-59. doi:10.1016/j.coph.2021.11.002
Avian Cell Line DuckCelt®-T17 Is an Efficient Production System for Live-Attenuated Human Metapneumovirus Vaccine Candidate Metavac®.
Chupin C, Pizzorno A, Traversier A, Brun P, Ogonczyk-Makowska D, Padey B, Milesi C, Dulière V, Laurent E, Julien T, Galloux M, Lina B, Eléouët JF, Moreau K, Hamelin ME, Terrier O, Boivin G, Dubois J, Rosa-Calatrava M.
Vaccines (Basel). 2021 Oct 16;9(10):1190. doi: 10.3390/vaccines9101190. PMID: 34696298; PMCID: PMC8540687.
Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs.
Lopez J, Mommert M, Mouton W, Pizzorno A, Brengel-Pesce K, Mezidi M, Villard M, Lina B, Richard JC, Fassier JB, Cheynet V, Padey B, Duliere V, Julien T, Paul S, Bastard P, Belot A, Bal A, Casanova JL, Rosa-Calatrava M, Morfin F, Walzer T, Trouillet-Assant S.
J Exp Med. 2021 Oct 4;218(10):e20211211. doi: 10.1084/jem.20211211. Epub 2021 Aug 6. Erratum in: J Exp Med. 2021 Oct 4;218(10): PMID: 34357402; PMCID: PMC8352718.
Structural insight into SARS-CoV-2 neutralizing antibodies and modulation of syncytia.
Asarnow D, Wang B, Lee WH, Hu Y, Huang CW, Faust B, Ng PML, Ngoh EZX, Bohn M, Bulkley D, Pizzorno A, Ary B, Tan HC, Lee CY, Minhat RA, Terrier O, Soh MK, Teo FJ, Yeap YYC, Seah SGK, Chan CEZ, Connelly E, Young NJ, Maurer-Stroh S, Renia L, Hanson BJ, Rosa-Calatrava M, Manglik A, Cheng Y, Craik CS, Wang CI.
Cell. 2021 Jun 10;184(12):3192-3204.e16. doi: 10.1016/j.cell.2021.04.033. Epub 2021 Apr 24. PMID: 33974910; PMCID: PMC8064868.
Transcriptional Profiling of Immune and Inflammatory Responses in the Context of SARS-CoV-2 Fungal Superinfection in a Human Airway Epithelial Model.
Nicolas de Lamballerie C, Pizzorno A, Fouret J, Szpiro L, Padey B, Dubois J, Julien T, Traversier A, Dulière V, Brun P, Lina B, Rosa-Calatrava M, Terrier O.
Microorganisms. 2020 Dec 11;8(12):1974. doi: 10.3390/microorganisms8121974.
In Vitro Combinations of Baloxavir Acid and Other Inhibitors against Seasonal Influenza A Viruses.
Checkmahomed L, Padey B, Pizzorno A, Terrier O, Rosa-Calatrava M, Abed Y, Baz M, Boivin G.
Viruses. 2020 Oct 8;12(10):1139. doi: 10.3390/v12101139.
In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2.
Pizzorno A, Padey B, Dubois J, Julien T, Traversier A, Dulière V, Brun P, Lina B, Rosa-Calatrava M, Terrier O.
Antiviral Res. 2020 Sep;181:104878. doi: 10.1016/j.antiviral.2020.104878. Epub 2020 Jul 15.
Human Respiratory Syncytial Virus-induced immune signature of infection revealed by transcriptome analysis of clinical pediatric nasopharyngeal swab samples.
Nicolas de Lamballerie C, Pizzorno A, Dubois J, Padey B, Julien T, Traversier A, Carbonneau J, Orcel E, Lina B, Hamelin ME, Roche M, Textoris J, Boivin G, Legras-Lachuer C, Terrier O, Rosa-Calatrava M.
J Infect Dis. 2020 Jul 29;jiaa468. doi: 10.1093/infdis/jiaa468. Online ahead of print.
Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia.
Pizzorno A, Padey B, Julien T, Trouillet-Assant S, Traversier A, Errazuriz-Cerda E, Fouret J, Dubois J, Gaymard A, Lescure FX, Dulière V, Brun P, Constant S, Poissy J, Lina B, Yazdanpanah Y, Terrier O, Rosa-Calatrava M.
Cell Rep Med. 2020 Jul 21;1(4):100059. doi: 10.1016/j.xcrm.2020.100059.
Novel calixarene-based surfactant enables low dose split inactivated vaccine protection against influenza infection.
Mandon ED, Pizzorno A, Traversier A, Champagne A, Hamelin ME, Lina B, Boivin G, Dejean E, Rosa-Calatrava M, Jawhari A.
Vaccine. 2020 Jan 10;38(2):278-287. doi: 10.1016/j.vaccine.2019.10.018. Epub 2019 Oct 18.
Advances in Influenza Virus-Like Particles bioprocesses.
Durous L, Rosa-Calatrava M and Petiot E.
Expert Rev Vaccines. 2019 Dec 12. doi: 10.1080/14760584.2019.1704262.
Strain-Dependent Impact of G and SH Deletions Provide New Insights for Live-Attenuated HMPV Vaccine Development.
Dubois J, Pizzorno A, Cavanagh MH, Padey B, Nicolas de Lamballerie C, Uyar O, Venable MC, Carbonneau J, Traversier A, Julien T, Lavigne S, Couture C, Lina B, Hamelin ME, Terrier O, Rosa-Calatrava M and Boivin G.
Vaccines 2019, 7(4), 164; https://doi.org/10.3390/vaccines7040164 Published: 30 October 2019.
Novel calixarene-based surfactant enables low dose split inactivated vaccine protection against influenza infection.
Mandon ED, Pizzorno A, Traversier A, Champagne A, Hamelin ME, Lina B, Boivin G, Dejean E, Rosa-Calatrava M, Jawhari A.
Vaccine. 2019 Oct 17. pii: S0264-410X(19)31381-7. doi: 10.1016/j.vaccine.2019.10.018.
Characterization of cellular transcriptomic signatures induced by different respiratory viruses in human reconstituted airway epithelia.
Nicolas de Lamballerie C, Pizzorno A, Dubois J, Julien T, Padey B, Bouveret M, Traversier A, Legras-Lachuer C, Lina B, Boivin G, Terrier O & Rosa-Calatrava C.
Scientific Reports. 2019 Aug 7; 9: 11493. doi: 10.1038/s41598-019-48013.
Toll-like receptor 5 agonist flagellin reduces influenza A virus replication independently of type I interferon and interleukin 22 and improves antiviral efficacy of oseltamivir.
Georgel AF, Cayet D, Pizzorno A, Rosa-Calatrava M, Paget C, Sencio V, Dubuisson J, Trottein F, Sirard JC, Carnoy C.
Antiviral Res. 2019 May 9;168:28-35. doi: 10.1016/j.antiviral.2019.05.002.
SPRi-based hemagglutinin quantitative assay for influenza vaccine production monitoring.
Durous L, Julien T, Padey B, Traversier A, Rosa-Calatrava M, Blum LJ, Marquette CA, Petiot E.
Vaccine. 2019 Mar 14;37(12):1614-1621. doi: 10.1016/j.vaccine.2019.01.083. Epub 2019 Feb 14.
Repurposing of drugs as novel influenza inhibitors from clinical gene expression infection signatures.
Pizzorno A,Terrier O, Nicolas de Lamballerie C, Julien T, Padey B, Traversier A, Roche M, Hamelin ME, Rheaume C, Croze S, Escuret V, Poissy J, Lina B, Legras-Lachuer C, Textoris J, Boivin G and Rosa-Calatrava M.
Front Immunol. 2019 Jan 29;10:60. doi: 10.3389/fimmu.2019.00060. eCollection 2019.
Expression and purification of native and functional influenza A virus matrix 2 proton selective ion channel.
Desuzinge Mandon E, Traversier A, Champagne A, Bernier L, Audebert S, Balme S, Dejean E, Rosa-Calatrava M and A Jawhari.
Protein Expression and Purification. 2016 Nov 5;131:42-50. doi: 10.1016/j.pep.2016.11.001.
Nucleolin interacts with influenza A nucleoprotein and contributes to viral ribonucleoprotein complexes nuclear trafficking and efficient influenza viral replication.
Terrier O, Carron C, De Chassey B, Dubois J, Traversier A, Julien T, Cartet G, Proust A, Hacot S, Ressnikof D, Lotteau V, Lina B, Diaz JJ, Moules V and Rosa-Calatrava M.
Scientific Report. 2016 Jul 4;6:29006. doi: 10.1038/srep29006.
Selective packaging of the influenza A genome and consequences for genetic reassortment.
Gerber M, Isel C, Moules V, Marquet R.
Trends Microbiol. 2014 Aug;22(8):446-55. doi: 10.1016/j.tim.2014.04.001. Epub 2014 May 2.
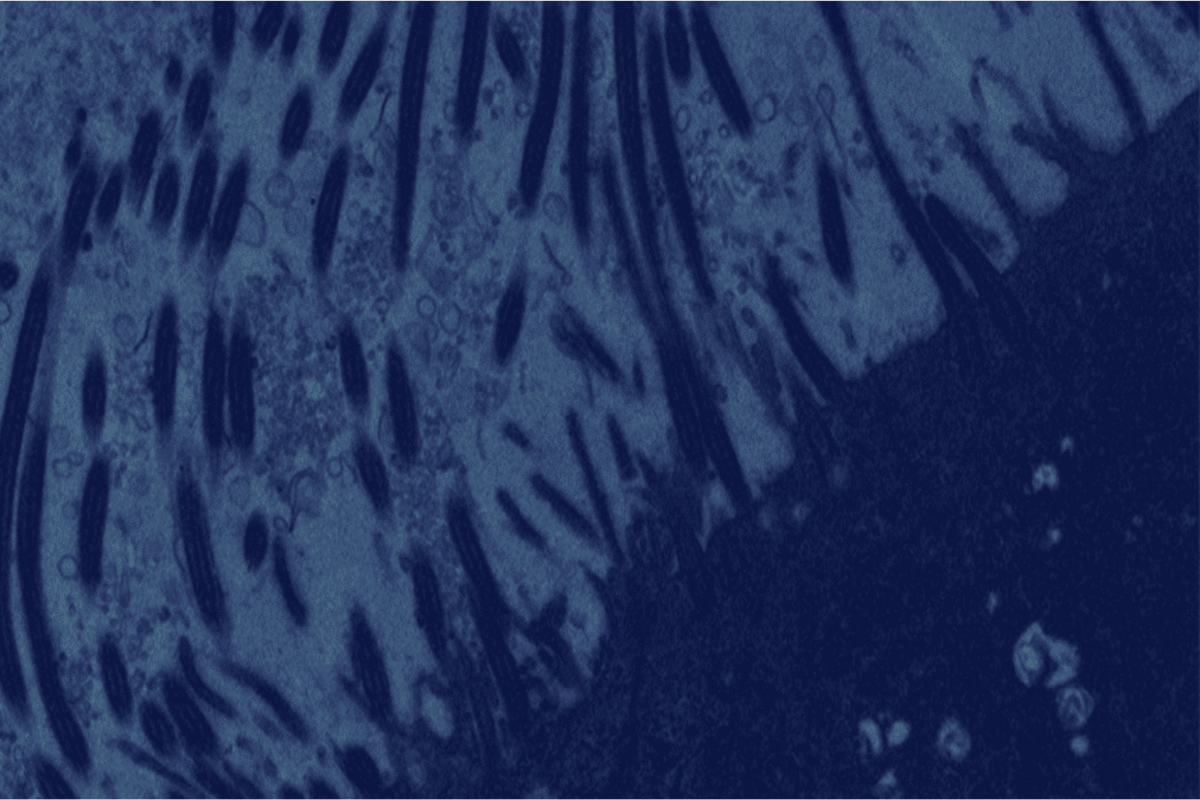
Ultrastructural fingerprints of avian influenza A (H7N9) virus in infected human lung cells.
Terrier O, Carron C, Cartet G, Traversier A, Julien T, Valette M, Lina B, Moules V, Rosa-Calatrava M.
Virology. 2014 May;456-457:39-42. doi: 10.1016/j.virol.2014.03.013. Epub 2014 Mar 28.
Critical role of segment-specific packaging signals in genetic reassortment of influenza A viruses.
Essere B, Yver M, Gavazzi C, Terrier O, Isel C, Fournier E, Giroux F, Textoris J, Julien T, Socratous C, Rosa-Calatrava M, Lina B, Marquet R, Moules V.
Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):E3840-8. doi: 10.1073/pnas.1308649110. Epub 2013 Sep 16.
Innovative germicidal UV and photocatalytic system dedicated to aircraft cabin eliminates volatile organic compounds and pathogenic micro-organisms.
Gorvel L, Yver M, Robert E, Harmant M, Rosa-Calatrava M, Lina B, Pierre Gorvel JP, Moulès V, Albalate R, Gaüzère C.
CLEAN – Soil, Air, WaterVolume 42, Issue 6, Article first published online: 20 OCT 2013. doi: 10.1002/clen.201300085